Patient history
An 81-year-old woman presented with claudication of the right leg.
Procedure summary
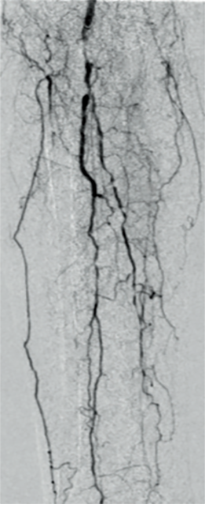
Angiography showed a flush occlusion at the bifurcation between the deep femoral artery (DFA) and superficial femoral artery (SFA). After crossing, control angiography showed a diffuse, sub-occlusive long SFA lesion (Figure 1).
Pre-dilatation was performed with plain angioplasty balloon. Next, one long-length Paclitaxel-coated Passeo-18 Lux DCB (6 x 200 mm) was inflated for three minutes to treat the segment, followed by a Pulsar-18 T3 BMS.
Results
The final angiography showed complete revascularization of the femoropopliteal segment (Figure 2).








 Femoropopliteal3
Femoropopliteal3
 Critical limb ischemia8
Critical limb ischemia8
 Below the knee9
Below the knee9
 Diabetes mellitus10
Diabetes mellitus10
 In-stent restenosis3
In-stent restenosis3