Prospective, international, multicenter, single-arm study with up to 60-month follow-up evaluating the
safety and efficacy of Dynetic-35 in the treatment of peripheral artery disease in the iliac arteries.

N = 160 subjects
Austria, Belgium,
France, Germany,
Hungary, Latvia

Primary Endpoint:
Major adverse events§ 12 months
post-index procedure
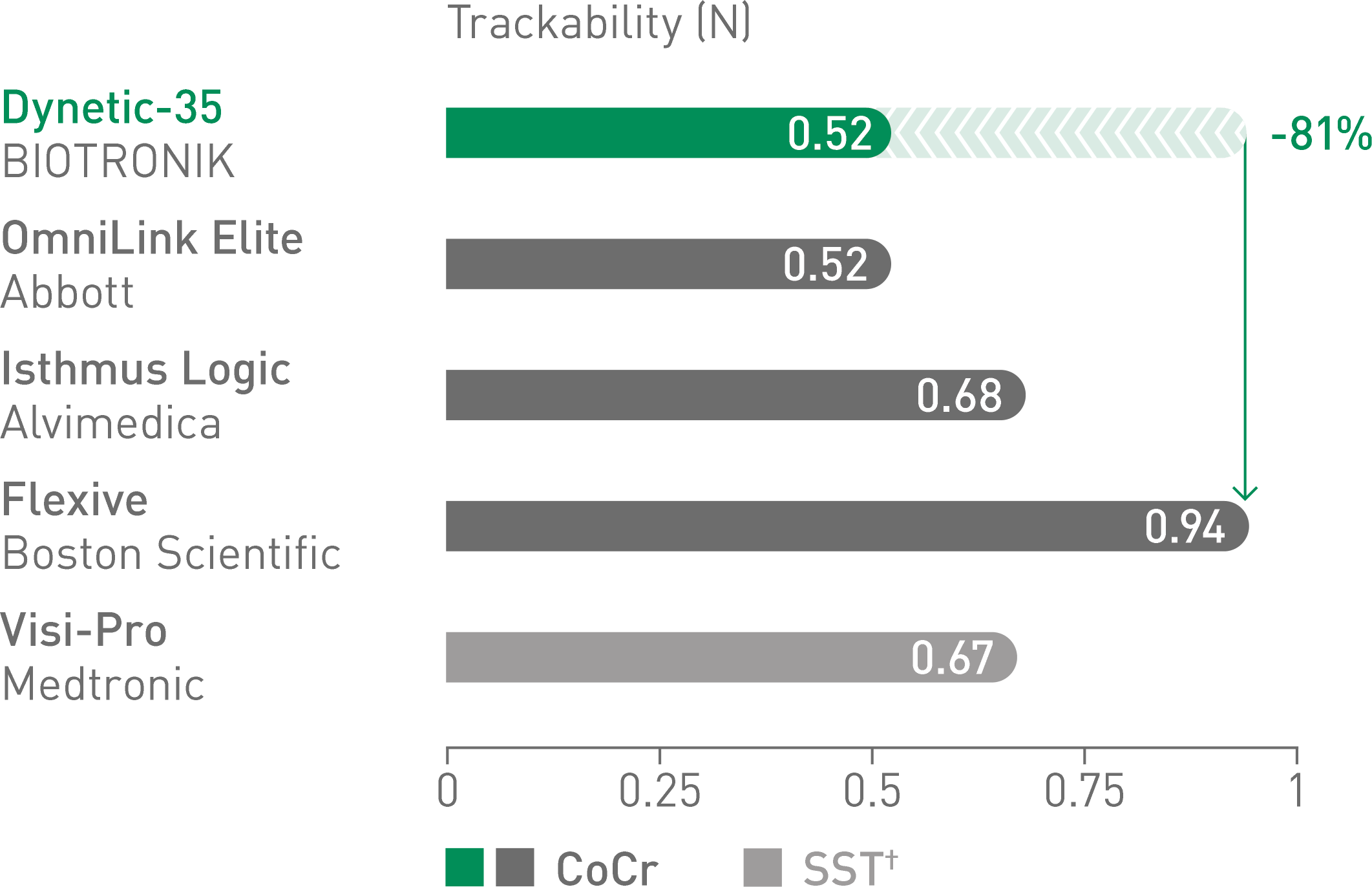
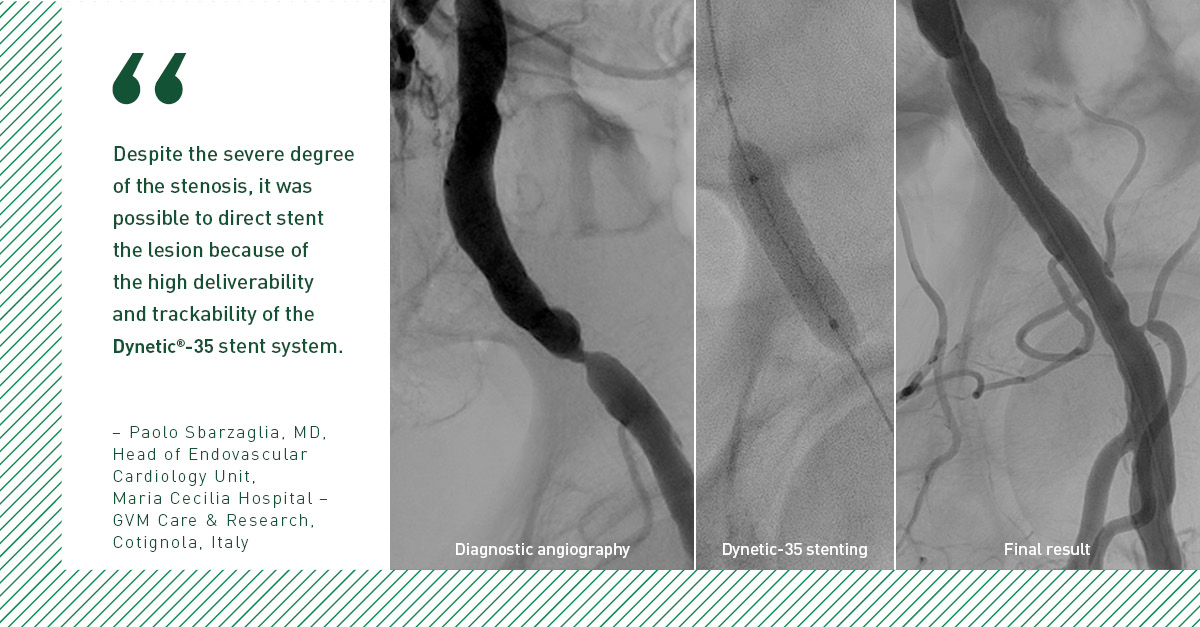
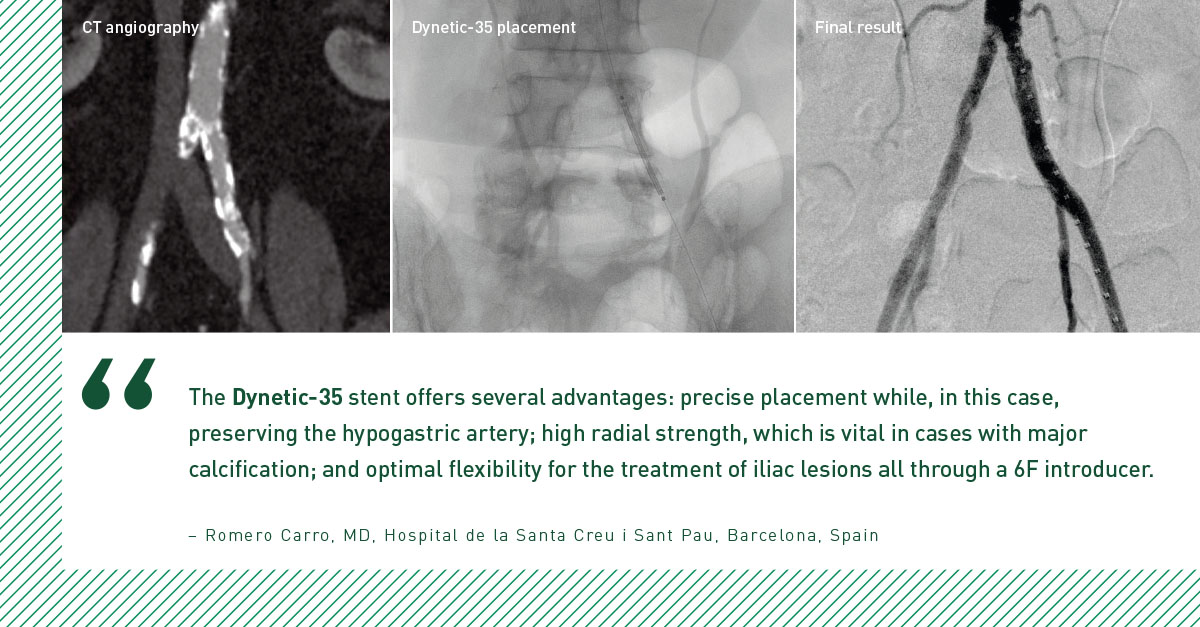
The 24-month BIONETIC-I study results support the long-term safety and effectiveness of the Dynetic-35 Cobalt Chromium Balloon-Expandable Stent System in the treatment of iliac artery disease.6
§Major adverse events includes device or procedure related death within 30 days post index procedure, clinically driven target
lesion revascularization,
and major index limb amputation up to 12 months post index procedure.